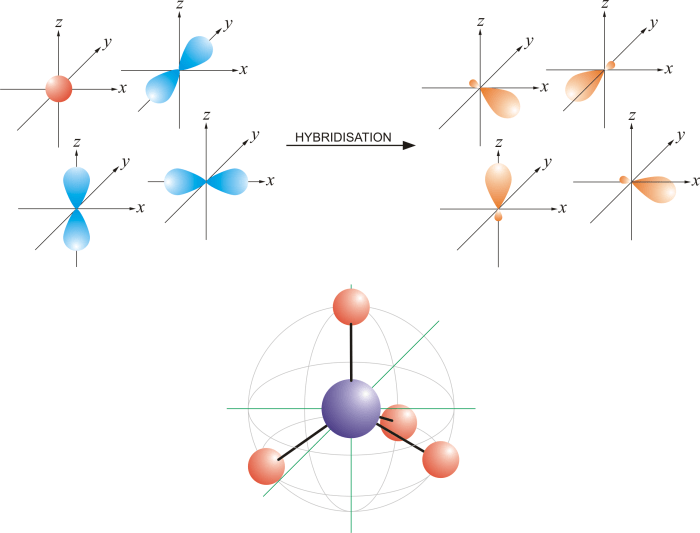
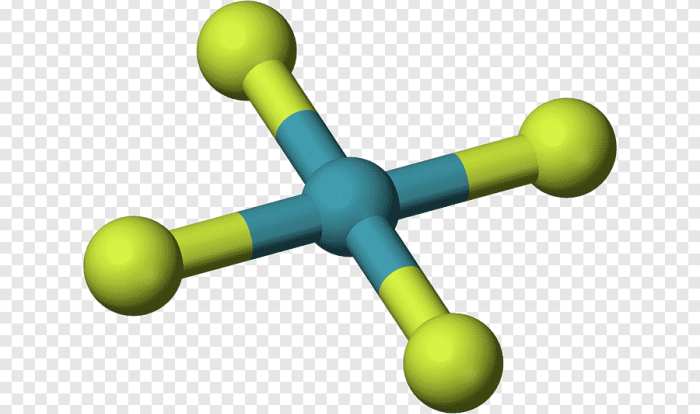
What is the orbital hybridization of the highlighted atom? This intriguing question unveils the intricacies of molecular geometry, guiding us through the captivating world of atomic orbitals and their transformative power. Delving into the depths of this concept, we embark on a journey to unravel the secrets that govern the shape and properties of molecules.
The significance of orbital hybridization extends far beyond mere theoretical understanding. It serves as a cornerstone in comprehending the behavior of molecules, predicting their reactivity, and unlocking the mysteries of chemical bonding. As we delve deeper into this realm, we will uncover the profound impact of orbital hybridization on our understanding of the chemical world.
Overview of Orbital Hybridization
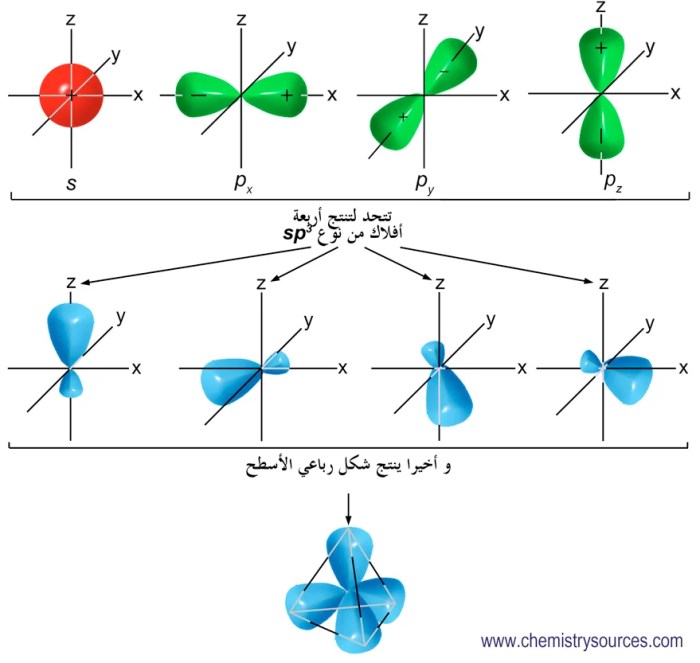
Orbital hybridization is a fundamental concept in chemistry that describes the process of combining atomic orbitals to form new hybrid orbitals with different shapes and energies. This process plays a crucial role in determining the molecular geometry and properties of compounds.
Atomic orbitals are the wave functions that describe the probability of finding an electron in a specific region around the nucleus. Each type of atomic orbital has a unique shape and energy level. The most common atomic orbitals are the s, p, and d orbitals.
Identifying the Highlighted Atom, What is the orbital hybridization of the highlighted atom
To determine the orbital hybridization of an atom, it is first necessary to identify the highlighted atom in the molecule. This is typically the atom that is central to the molecule or has the most bonds to other atoms.
It is important to consider the atom’s position and bonding environment when identifying the highlighted atom. For example, in a molecule with a carbon-carbon double bond, the carbon atom that is double-bonded to another carbon atom is the highlighted atom.
Determining the Orbital Hybridization of the Highlighted Atom
Once the highlighted atom has been identified, the next step is to determine its orbital hybridization. This can be done by following a series of steps:
- Count the number of electron groups around the highlighted atom. Electron groups include lone pairs and bonds to other atoms.
- Determine the hybridization based on the number of electron groups. The following table shows the relationship between the number of electron groups and the hybridization:
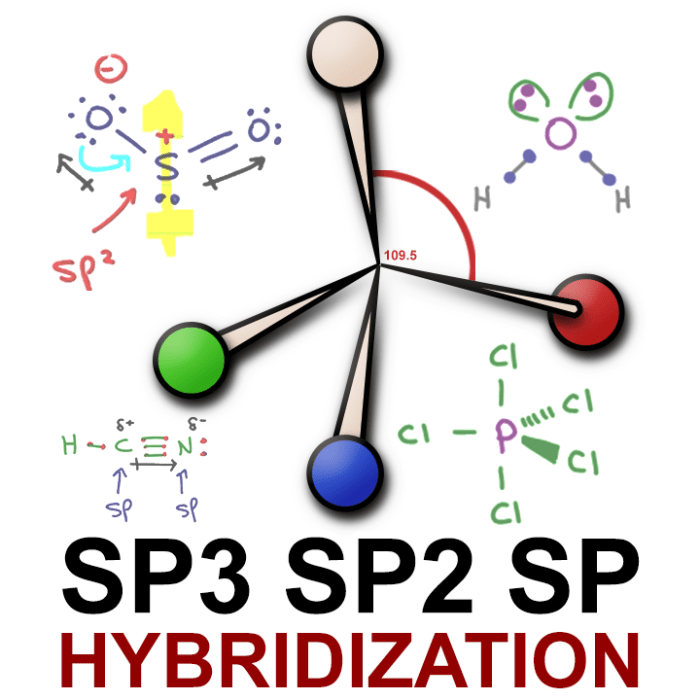
Number of Electron Groups Hybridization 2 sp 3 sp2 4 sp3 5 sp3d 6 sp3d2
Examples of Orbital Hybridization
The following table provides examples of molecules with different orbital hybridization schemes:
| Highlighted Atom | Hybridization | Molecular Geometry |
|---|---|---|
| Carbon in methane (CH4) | sp3 | Tetrahedral |
| Carbon in ethene (C2H4) | sp2 | Trigonal planar |
| Nitrogen in ammonia (NH3) | sp3 | Trigonal pyramidal |
| Oxygen in water (H2O) | sp3 | Bent |
Applications of Orbital Hybridization
Orbital hybridization has numerous applications in understanding molecular properties. It can be used to predict the bond length, bond angle, and molecular shape of a compound.
For example, in a molecule with sp 3hybridized carbon atoms, the bond angles are typically 109.5 degrees. This is because the sp 3hybrid orbitals are tetrahedral in shape, which results in a tetrahedral molecular geometry.
Advanced Concepts
In addition to the basic concepts of orbital hybridization, there are also more advanced concepts that can be explored.
One such concept is resonance. Resonance occurs when a molecule can be represented by multiple Lewis structures. In these cases, the actual structure of the molecule is a hybrid of the resonance structures.
Another advanced concept is hyperconjugation. Hyperconjugation is a type of resonance that occurs when a sigma bond interacts with a pi bond. This can lead to the stabilization of the molecule.
Essential FAQs: What Is The Orbital Hybridization Of The Highlighted Atom
What is the significance of orbital hybridization in determining molecular geometry?
Orbital hybridization plays a crucial role in determining molecular geometry by dictating the arrangement of electron pairs around the central atom. The hybridization scheme influences the shape and orientation of the orbitals, ultimately shaping the overall geometry of the molecule.
How can we identify the highlighted atom in a given molecule?
Identifying the highlighted atom requires careful examination of the molecular structure. Typically, the highlighted atom is the central atom or the atom of interest around which the other atoms are arranged. Its position and bonding environment provide valuable clues for accurate identification.
What are the steps involved in determining the orbital hybridization of the highlighted atom?
Determining the orbital hybridization of the highlighted atom involves a systematic approach. First, count the total number of valence electrons for the atom. Next, consider the number of electron pairs involved in bonding with other atoms. Based on these factors, determine the hybridization scheme that best accommodates the observed bonding pattern.