Stoichiometry mass volume particle practice answer key – Delving into the realm of stoichiometry, this comprehensive answer key unveils the intricate connections between mass, volume, and particles in chemical reactions. Through a series of carefully crafted examples, practice problems, and in-depth explanations, this guide empowers learners to navigate the complexities of stoichiometry with confidence and precision.
As we embark on this journey, we will explore the fundamental concepts of stoichiometry, unraveling the secrets of balanced chemical equations and their applications in determining mass and volume relationships. We will delve into the intricacies of mass-to-mass, volume-to-volume, and mass-to-volume calculations, gaining mastery over the conversion between different units of measurement.
Stoichiometry: Mass and Volume Relationships

Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It is used to determine the amount of reactants and products that are involved in a reaction, as well as the mass and volume relationships between them.
Balanced chemical equations are used to represent chemical reactions. A balanced chemical equation shows the chemical formulas of the reactants and products, as well as the coefficients that indicate the number of moles of each reactant and product that are involved in the reaction.
For example, the following equation shows the combustion of methane:
CH4+ 2O 2→ CO 2+ 2H 2O
This equation shows that one mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide and two moles of water.
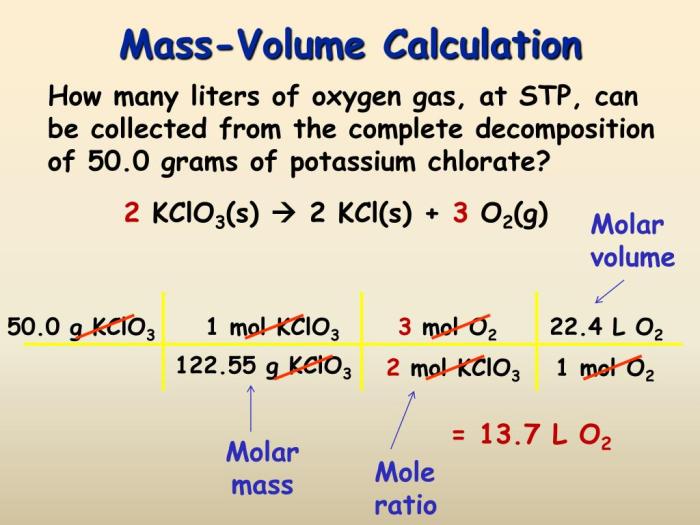
Mass-to-Mass Calculations, Stoichiometry mass volume particle practice answer key
Mass-to-mass calculations are used to convert between the masses of reactants and products in a balanced chemical equation. To perform a mass-to-mass calculation, you need to know the following:
- The balanced chemical equation for the reaction
- The molar mass of each reactant and product
Once you have this information, you can use the following steps to perform a mass-to-mass calculation:
- Convert the mass of the known reactant or product to moles.
- Use the coefficients in the balanced chemical equation to determine the moles of the other reactant or product.
- Convert the moles of the other reactant or product to mass.
Volume-to-Volume Calculations
Volume-to-volume calculations are used to convert between the volumes of gases in a balanced chemical equation. To perform a volume-to-volume calculation, you need to know the following:
- The balanced chemical equation for the reaction
- The molar volume of each gas
Once you have this information, you can use the following steps to perform a volume-to-volume calculation:
- Convert the volume of the known gas to moles.
- Use the coefficients in the balanced chemical equation to determine the moles of the other gas.
- Convert the moles of the other gas to volume.
FAQ Compilation: Stoichiometry Mass Volume Particle Practice Answer Key
What is stoichiometry?
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions.
How can I use stoichiometry to determine the mass of a product?
To determine the mass of a product, you can use the mole ratios from a balanced chemical equation and the molar mass of the product.
What is the difference between a mass-to-mass calculation and a volume-to-volume calculation?
A mass-to-mass calculation involves converting between the masses of reactants and products, while a volume-to-volume calculation involves converting between the volumes of gases in a reaction.