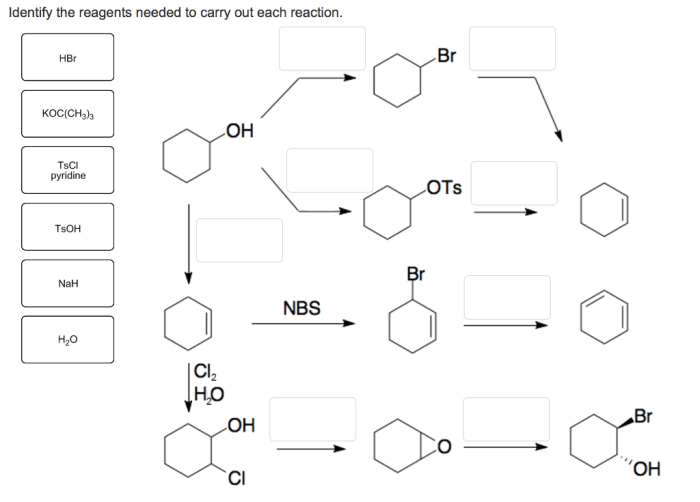
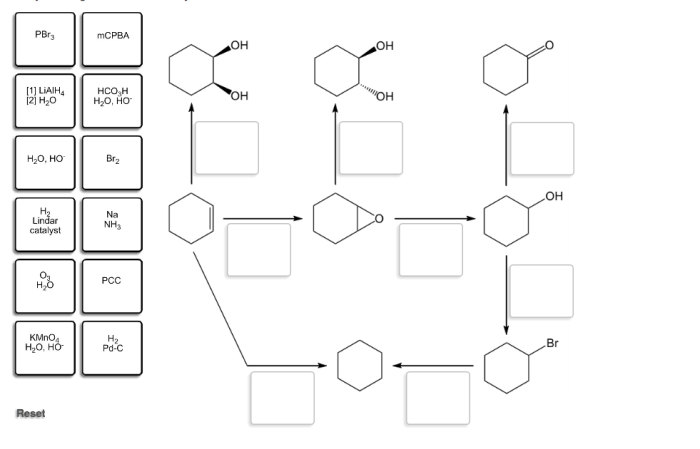
Identify the reagents needed to carry out each reaction – In the realm of chemistry, the identification of reagents plays a pivotal role in orchestrating successful reactions. Reagents, the essential building blocks of chemical transformations, dictate the course and outcome of these intricate processes. This guide delves into the multifaceted world of reagents, empowering you with the knowledge to select and employ them effectively for your experimental endeavors.
We will embark on a journey to unravel the concept of reagents, exploring their diverse types and functions. We will uncover the significance of reaction conditions, unravel the intricacies of reaction pathways, and delve into strategies for optimizing reaction yields.
Throughout this exploration, we will encounter illuminating examples and practical applications that will solidify your understanding of this fundamental aspect of chemistry.
Identify the Reagents
Reagents are chemical substances that are used to bring about a chemical reaction. They can be classified into two main types: reactants and catalysts. Reactants are the substances that are consumed in the reaction, while catalysts are substances that participate in the reaction but are not consumed.
The choice of reagents is critical for the success of a chemical reaction. The reagents must be compatible with each other and must be able to react under the desired conditions. The concentration of the reagents is also important, as it can affect the rate of the reaction.
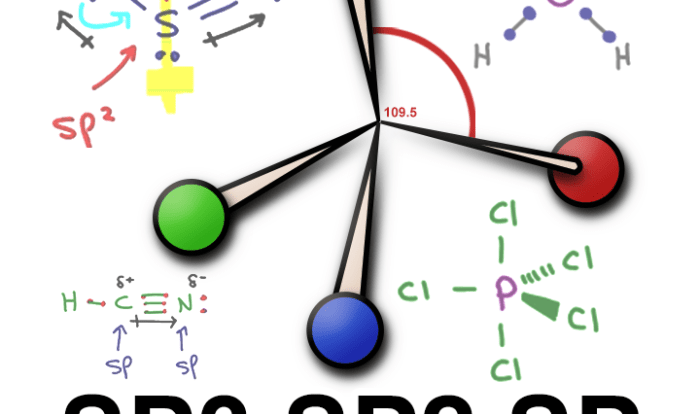
Common reagents include acids, bases, oxidizing agents, and reducing agents. Acids are substances that donate protons, while bases are substances that accept protons. Oxidizing agents are substances that cause other substances to lose electrons, while reducing agents are substances that cause other substances to gain electrons.
Determine Reaction Conditions
Reaction conditions are the environmental factors that affect the rate and outcome of a chemical reaction. The most important reaction conditions are temperature, pressure, and pH.
Temperature affects the rate of a reaction by increasing the kinetic energy of the reactants. This makes it more likely that the reactants will collide with each other and react. Pressure affects the rate of a reaction by increasing the concentration of the reactants.
This makes it more likely that the reactants will collide with each other and react.
pH affects the rate of a reaction by changing the ionization state of the reactants. This can change the reactivity of the reactants and make them more or less likely to react.
Design Reaction Pathways: Identify The Reagents Needed To Carry Out Each Reaction

A reaction pathway is a step-by-step description of how a chemical reaction occurs. Reaction pathways are important because they allow chemists to understand the mechanism of a reaction and to predict the products that will be formed.
To design a reaction pathway, chemists must first identify the reactants and the products of the reaction. They must then determine the intermediate steps that occur between the reactants and the products. The intermediate steps are often unstable compounds that cannot be isolated.
Once the reaction pathway has been determined, chemists can use it to predict the products that will be formed and to optimize the reaction conditions.
Optimize Reaction Yields
Reaction yield is the amount of product that is formed in a chemical reaction. The yield of a reaction can be affected by a number of factors, including the choice of reagents, the reaction conditions, and the presence of impurities.
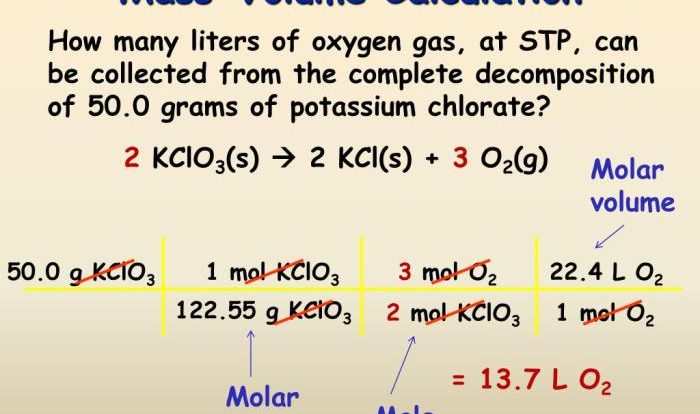
To optimize reaction yields, chemists must first identify the limiting reagent. The limiting reagent is the reactant that is consumed first in the reaction. Once the limiting reagent has been identified, chemists can adjust the reaction conditions to maximize the yield of the product.
Chemists can also use stoichiometry to calculate the theoretical yield of a reaction. The theoretical yield is the maximum amount of product that can be formed in a reaction. The actual yield is often less than the theoretical yield due to side reactions and other losses.
Query Resolution
What are the different types of reagents?
Reagents encompass a wide range of substances, including catalysts, acids, bases, oxidizing agents, reducing agents, and solvents.
How do I determine the reaction conditions for a specific reaction?
Reaction conditions vary depending on the specific reaction being carried out. Factors to consider include temperature, pressure, pH, and the presence of catalysts.
What is the importance of optimizing reaction yields?
Optimizing reaction yields ensures efficient use of reagents and minimizes waste. It involves selecting appropriate reagents, optimizing reaction conditions, and calculating stoichiometric ratios.