A solution containing HCl and the weak acid HClO2 presents a fascinating realm of chemical interactions, where the interplay of acid-base and redox reactions unveils a multitude of intriguing phenomena. This solution exhibits unique properties that influence chemical reactions and offers a valuable tool for various applications.
The chemical properties of this solution arise from the presence of HCl, a strong acid, and HClO2, a weak acid. The contrasting strengths of these acids lead to a dynamic equilibrium, affecting the solution’s pH and reactivity. Understanding the behavior of this solution requires an in-depth exploration of the acid-base reactions that govern its chemistry.
Solution Properties
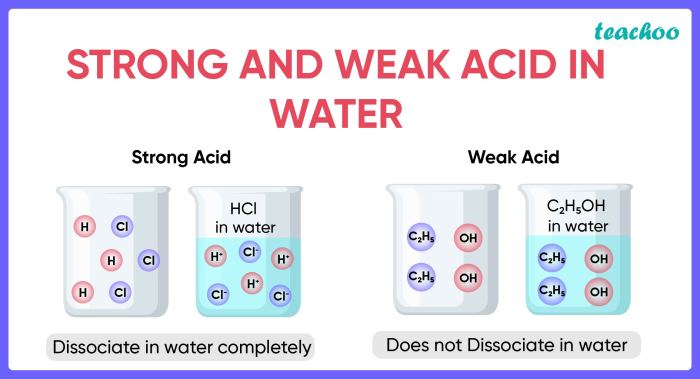
A solution containing HCl and HClO2 exhibits unique chemical properties due to the presence of both a strong acid (HCl) and a weak acid (HClO2). The strong acid HCl completely dissociates in water, releasing H+ ions, while the weak acid HClO2 partially dissociates, releasing H+ and ClO2- ions.
The presence of H+ ions in the solution contributes to its acidic nature. The solution has a low pH value, indicating a high concentration of H+ ions. The weak acid HClO2, on the other hand, undergoes proton transfer reactions, resulting in the formation of H2O and ClO2-.
This equilibrium reaction affects the pH of the solution and can influence chemical reactions that occur within it.
Acid-Base Reactions
In a solution containing HCl and HClO2, acid-base reactions can occur between the two acids and other species present in the solution. HCl, being a strong acid, can donate protons to weak bases, leading to the formation of conjugate acid-base pairs.
HClO2, on the other hand, can act as both an acid and a base, depending on the pH of the solution and the species it reacts with.
The equilibrium constant for the dissociation of HClO2 determines the extent to which it donates or accepts protons. Factors such as temperature and the presence of other ions in the solution can affect the equilibrium position, influencing the pH and the reactivity of the solution.
Redox Reactions, A solution containing hcl and the weak acid hclo2
Redox reactions involve the transfer of electrons between species. In a solution containing HCl and HClO2, redox reactions can occur under specific conditions. HClO2 can undergo reduction, gaining electrons to form HClO or Cl-. The reduction of HClO2 can be facilitated by the presence of reducing agents, such as metals or other reducing species.
The oxidation of HClO2 can also occur, resulting in the formation of ClO2 or ClO3-. Oxidizing agents, such as hydrogen peroxide or potassium permanganate, can drive the oxidation of HClO2. The specific redox reactions that occur depend on the pH of the solution, the presence of other species, and the redox potentials of the reactants and products.
Spectroscopic Analysis
Spectroscopic techniques provide valuable insights into the composition and properties of a solution containing HCl and HClO2. UV-Vis spectroscopy can be used to determine the concentration of HClO2 in the solution based on its characteristic absorption bands. IR spectroscopy can identify the presence of functional groups, such as the O-H and C-O bonds in HClO2.
NMR spectroscopy can provide information about the molecular structure and dynamics of HClO2. The chemical shifts and coupling constants obtained from NMR spectra can help elucidate the bonding environment and conformational changes of HClO2 in the solution.
Applications
Solutions containing HCl and HClO2 find applications in various fields, including:
- Industry:HCl is widely used in the production of fertilizers, plastics, and other chemicals. HClO2 is used as a bleaching agent in the paper and textile industries.
- Research:Solutions containing HCl and HClO2 are used in laboratory research to study acid-base reactions, redox reactions, and other chemical processes.
- Medicine:Dilute solutions of HCl are used as a digestive aid and to treat certain medical conditions. HClO2 has antibacterial and antiviral properties and is used as a disinfectant and antiseptic.
FAQ Overview: A Solution Containing Hcl And The Weak Acid Hclo2
What is the pH of a solution containing HCl and HClO2?
The pH of a solution containing HCl and HClO2 depends on the concentrations of both acids. HCl is a strong acid, while HClO2 is a weak acid. Therefore, the pH of the solution will be lower (more acidic) than 7.
What are the products of the reaction between HCl and HClO2?
The reaction between HCl and HClO2 produces Cl2 and H2O.
Can a solution containing HCl and HClO2 be used as a disinfectant?
Yes, a solution containing HCl and HClO2 can be used as a disinfectant. HCl is a strong acid that kills bacteria, and HClO2 is a weak acid that helps to remove organic matter.