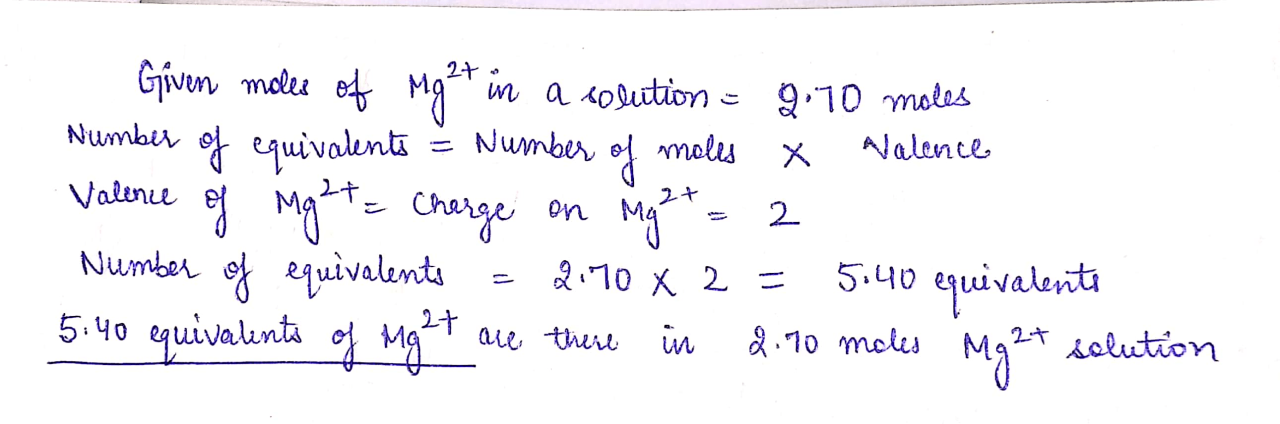How many equivalents are in 0.40 mole of K+? This question delves into the fundamental concepts of moles and equivalents in chemistry, exploring their relationship and significance in various chemical applications. Understanding equivalents is crucial for balancing chemical equations, determining reaction stoichiometry, and comprehending the behavior of ions in solution.
In this discussion, we will define the term “equivalent” in the context of chemistry, explain how equivalents relate to moles, and provide a step-by-step calculation to determine the number of equivalents present in 0.40 mole of K+.
Equivalents in 0.40 Moles of K+
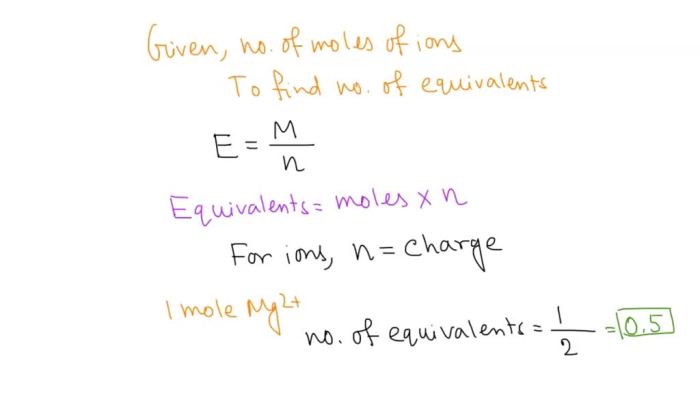
In chemistry, the mole is a unit of measurement that represents the amount of substance. One mole is equal to 6.022 × 10 23entities, which can be atoms, molecules, ions, or electrons. The equivalent is another unit of measurement that is used to express the amount of a substance that can react with or combine with a specific amount of another substance.
The given value is 0.40 moles of K+ ions. To determine the number of equivalents in this amount, we need to understand the concept of equivalents and how they relate to moles.
Definition of Equivalents
An equivalent is defined as the amount of a substance that can react with or combine with one mole of hydrogen ions (H+) or one mole of hydroxide ions (OH-). In other words, an equivalent is the amount of a substance that can donate or accept one mole of protons.
Equivalents are related to moles by the following equation:
Equivalents = Moles × Valence
where valence is the number of electrons that an atom or ion can donate or accept.
Calculating Equivalents from Moles, How many equivalents are in 0.40 mole of k+
To calculate the number of equivalents in 0.40 moles of K+ ions, we can use the formula:
Equivalents = Moles × Valence
The valence of K+ is 1, so the calculation becomes:
Equivalents = 0.40 moles × 1 = 0.40 equivalents
Significance of Equivalents
Equivalents are important in various chemical applications, including:
- Balancing chemical equations
- Determining reaction stoichiometry
- Calculating the concentration of solutions
- Understanding the reactivity of substances
By understanding the concept of equivalents, chemists can accurately represent the amount of a substance that is involved in a chemical reaction and predict the products that will be formed.
Questions Often Asked: How Many Equivalents Are In 0.40 Mole Of K+
What is the definition of an equivalent in chemistry?
An equivalent is the amount of a substance that can react with or combine with a specific amount of another substance.
How are equivalents related to moles?
The number of equivalents in a given amount of substance is equal to the number of moles multiplied by the substance’s equivalent weight.
What is the equivalent weight of K+?
The equivalent weight of K+ is 39.1 g/eq.