Beginning with the graphing periodic trends answer key, this comprehensive guide delves into the fascinating realm of periodic trends, empowering you with the knowledge to decipher these patterns and unlock their predictive power in chemistry.
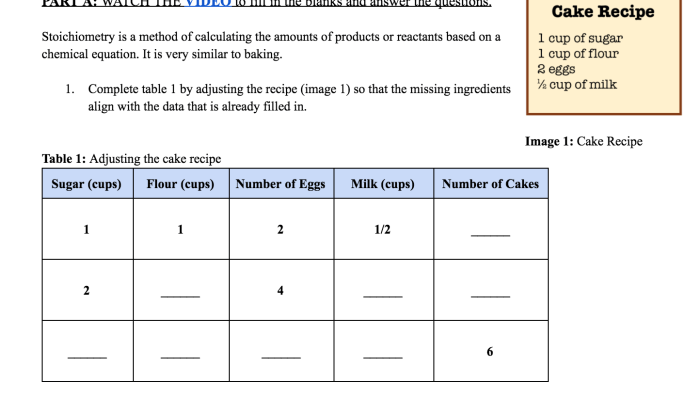
Periodic trends are the predictable changes in the properties of elements as you move across a period or down a group in the periodic table. Understanding these trends is crucial for comprehending the behavior of elements and predicting their chemical properties.
Introduction
Periodic trends refer to the observable patterns and variations in the chemical and physical properties of elements as they are arranged in the periodic table.
Understanding periodic trends is crucial in chemistry as it allows scientists to predict the behavior and properties of elements based on their position in the periodic table. This knowledge is essential for developing new materials, understanding chemical reactions, and advancing various fields of science and technology.
Methods for Graphing Periodic Trends
Periodic trends can be graphed in various ways, each providing different insights into the relationships between atomic properties and atomic number. The choice of graphing method depends on the specific trend being examined.
Line Graphs
Line graphs are used to show the continuous change in a periodic trend over the atomic number of the elements. The atomic number is plotted on the x-axis, and the trend value is plotted on the y-axis. Line graphs are useful for visualizing the overall trend and identifying any anomalies or discontinuities.
Bar Graphs
Bar graphs are used to compare the values of a periodic trend across different elements. The elements are plotted on the x-axis, and the trend value is plotted on the y-axis. Bar graphs are useful for comparing the relative magnitudes of the trend values and identifying any elements that deviate significantly from the overall trend.
Scatter Plots
Scatter plots are used to visualize the relationship between two periodic trends. The values of one trend are plotted on the x-axis, and the values of the other trend are plotted on the y-axis. Scatter plots can reveal correlations or relationships between different trends and identify any outliers.
Heat Maps
Heat maps are used to visualize the values of a periodic trend across a grid of elements. The elements are arranged in rows and columns, and the trend value is represented by a color gradient. Heat maps are useful for identifying patterns and trends in the values of a periodic trend and for comparing the values of the trend across different groups or periods of elements.
Key Features of Periodic Trend Graphs
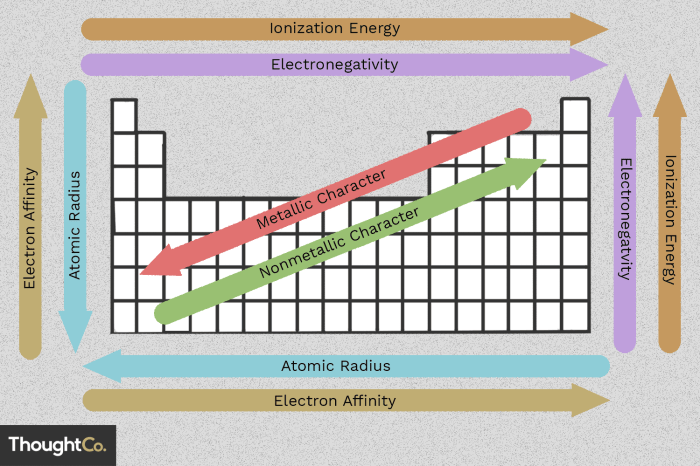
Periodic trend graphs are valuable tools for visualizing and understanding the periodic trends of elements. These graphs typically plot a chemical or physical property of an element against its atomic number or period. By examining these graphs, we can identify key features that provide insights into the underlying relationships between the elements.
One of the most important features of periodic trend graphs is the periodic repetition of properties. As we move across a period (row) in the periodic table, the properties of the elements change in a regular and predictable manner. This is because the number of electrons in the outermost energy level (valence electrons) increases by one for each element, leading to a gradual change in chemical and physical properties.
Another key feature of periodic trend graphs is the presence of maxima and minima. These are points on the graph where the property being plotted reaches its highest or lowest value. Maxima and minima can provide valuable information about the stability and reactivity of elements.
For example, elements with high ionization energies (a measure of the energy required to remove an electron) typically form stable ions and are less reactive, while elements with low ionization energies are more reactive.
Finally, periodic trend graphs often exhibit discontinuities. These are sharp changes in the property being plotted that occur at certain points in the periodic table. Discontinuities can indicate changes in the electronic configuration of the elements or the formation of new types of chemical bonds.
By understanding the key features of periodic trend graphs, we can gain valuable insights into the chemical and physical properties of elements. These graphs are essential tools for predicting the behavior of elements and understanding the underlying principles of chemistry.
Applications of Periodic Trend Graphs
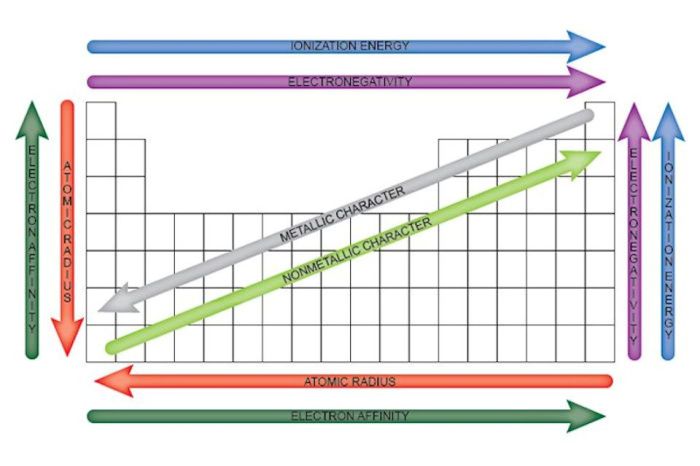
Periodic trend graphs are powerful tools that can be used to predict chemical properties. By understanding the trends in these graphs, chemists can make informed predictions about the behavior of elements and compounds.
Predicting Reactivity
One of the most important applications of periodic trend graphs is predicting reactivity. The reactivity of an element is a measure of how likely it is to undergo a chemical reaction. In general, the more reactive an element, the more likely it is to form compounds with other elements.
Periodic trend graphs can be used to predict the reactivity of an element by looking at its position on the periodic table. Elements in the same group (vertical column) tend to have similar reactivity. For example, all of the alkali metals (Group 1) are highly reactive.
All of the noble gases (Group 18) are unreactive.
Predicting Physical Properties
Periodic trend graphs can also be used to predict the physical properties of elements and compounds. Physical properties include things like melting point, boiling point, and density. By understanding the trends in these graphs, chemists can make informed predictions about the physical properties of a substance.
For example, the melting point of an element tends to increase as you move from left to right across a period. This is because the atoms in the elements on the right side of the periodic table have more protons and electrons than the atoms on the left side.
The increased number of protons and electrons results in stronger interatomic forces, which makes it more difficult to melt the element.
Limitations of Periodic Trend Graphs
Periodic trend graphs, while providing valuable insights, have certain limitations that should be acknowledged to avoid misinterpretations:
Data Limitations
- Missing Data:Some elements may not have complete data for all periodic trends, which can lead to gaps in the graphs.
- Exceptions and Anomalies:Certain elements may deviate from the general trends due to factors such as their electronic configurations or specific properties.
Graph Interpretation, Graphing periodic trends answer key
- Overgeneralization:Graphs may represent idealized trends that may not accurately reflect the behavior of all elements in all circumstances.
- Non-Linear Trends:Some periodic trends may not follow a linear or smooth curve, making it difficult to extrapolate values accurately.
Cautions
- Avoid Extrapolation:Extrapolating trends beyond the range of known data can lead to unreliable predictions.
- Consider Context:Periodic trends should be interpreted in the context of other chemical properties and factors.
Query Resolution: Graphing Periodic Trends Answer Key
What are the different methods for graphing periodic trends?
The two main methods are line graphs and bar graphs. Line graphs show the trend of a property as a function of atomic number, while bar graphs show the trend of a property as a function of group number.
What are the key features of periodic trend graphs?
The key features include peaks, valleys, and plateaus. Peaks and valleys indicate maximum and minimum values, respectively, while plateaus indicate a range of values where the property remains relatively constant.
What are the applications of periodic trend graphs?
Periodic trend graphs can be used to predict the properties of unknown elements, identify trends in chemical reactivity, and understand the electronic structure of elements.